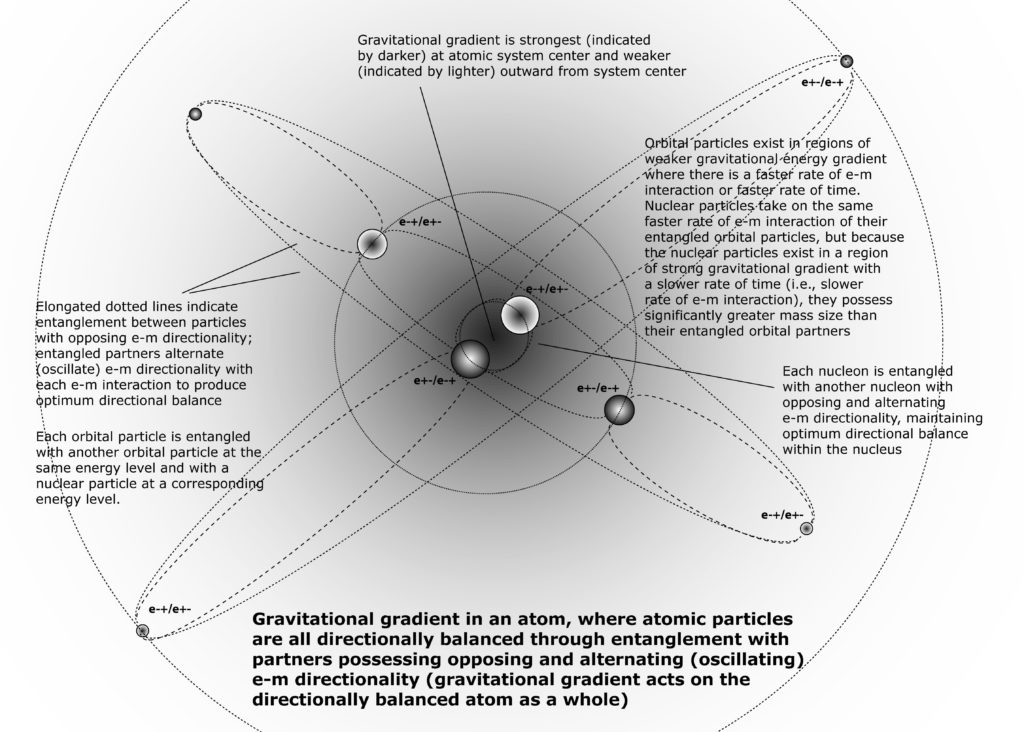
In this model, the atomic nucleus mimics its outer orbital structure. So energy level 1s2 in an outer orbital corresponds to a 1s2 energy level within the atomic nucleus. The two orbital particles in 1s2 are entangled with each other, and each of them is entangled with one nucleon occupying the corresponding 1s2 orbital in the nucleus. The two nucleons occupying 1s2 in the nucleus are also entangled with each other. So each orbital particle or nucleon is entangled with two other partners. It is likely that the two sets of three entangled particles are also entangled with each other, composing a total system of six entangled partners.
In this model, the atomic nucleus mimics its outer orbital structure. So energy level 1s2 in an outer orbital corresponds to a 1s2 energy level within the atomic nucleus. The two orbital particles in 1s2 are entangled with each other, and each of them is entangled with one nucleon occupying the corresponding 1s2 orbital in the nucleus. The two nucleons occupying 1s2 in the nucleus are also entangled with each other. So each orbital particle or nucleon is entangled with two other partners. It is likely that the two sets of three entangled particles are also entangled with each other, composing a total system of six entangled partners.
Entangled partners within an energy level act as pairs of entangled particles within each orbital, but also as one energy entity for that energy level. So if the 2s and 2p energy sublevels are filled with four pairs of entangled partners, it also acts as a single energy entity composed of eight entangled partners. This is significant in determining properties within the nucleus. In addition, nucleons possess different amounts of total mass-energy due to the much stronger energy gradient within the nucleus compared to the regions occupied by it orbital partners, and the rates of e-m interactions of the orbital particles.
Since the orbital particles exist in a much weaker gravitational energy gradient, they have a faster rate of e-m interaction. The corresponding nucleons exist in a much stronger gravitational energy gradient, and should have a slower rate of e-m interaction. However, because the orbital particles are each entangled with a nucleon, the nucleon takes on the same higher rate of e-m interaction for optimal directional balance, and because it exists in a stronger gravitational energy gradient with more potential (to kinetic energy) of space and a corresponding slower rate of time, it consists of significantly more mass-energy than that of its orbital partner.
This model assumes that each nucleon exists as a multiple of proton mass-energy, so that a nucleon may consist of 1 X proton mass, 2 X proton mass, or 3 X proton mass. It is possible that nucleons may take on other mass-energies, but to simplify for purposes of exploration and illustration, the above multiples of proton mass will be used for this model.
Since the particles occupying a given energy level, such as the 2s, 2p energy sublevels, may act as a single energy entity of 8 entangled particles for filled 2s, 2p orbitals, both individual particles and the single energy entity may have separate quantum properties. Spin is the quantum property that is considered in this model. In essence, the single energy entity composed of the total number of particles (whether in the nucleus or in the outer orbitals) of a given energy level form a separate energy structure with its own quantum properties, including spin.
This model uses this concept to determine whether it is possible for an atomic nucleus to be composed of nucleons that do not include neutrons. Nucleon spin of each isotope is identified as a multiple of spin 1/2 (fermion) or as a multiple of a whole number (boson). See Rules for Nucleon Structures.