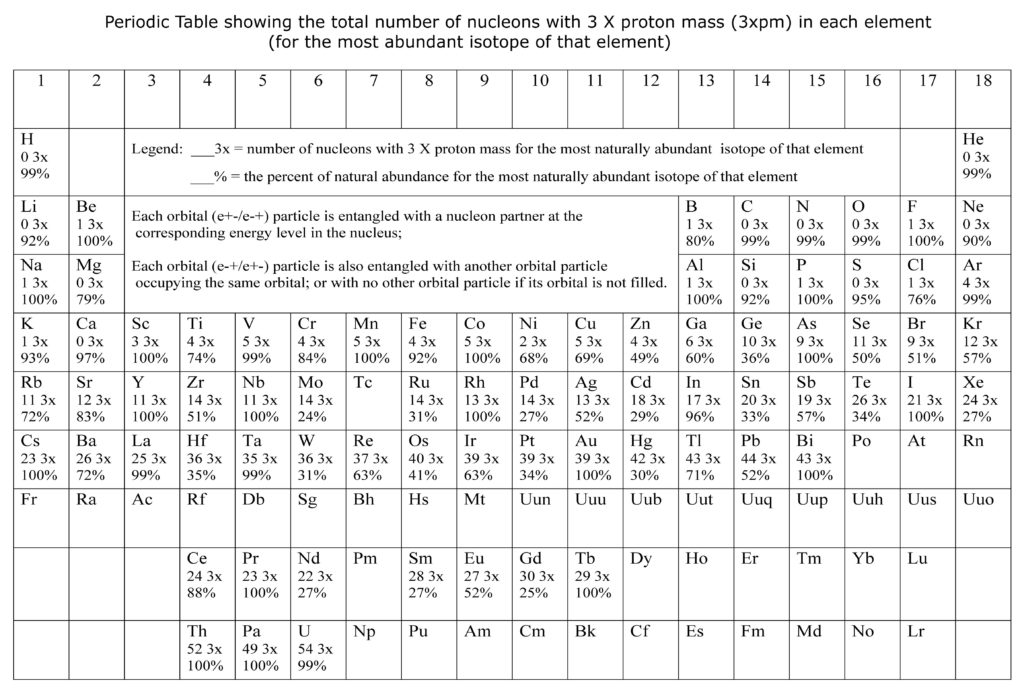
The Periodic Table below lists the number of nucleons that are 3 X the mass of a proton (3xpm) for the most naturally abundant isotope of each element. The per cent natural abundance for each of the most naturally occurring isotope is also given. Almost all other nucleons are 2 X the mass of a proton (2xpm).
The Periodic Table below lists the number of nucleons that are 3 X the mass of a proton (3xpm) for the most naturally abundant isotope of each element. The per cent natural abundance for each of the most naturally occurring isotope is also given. Almost all other nucleons are 2 X the mass of a proton (2xpm).